Our expert teams work closely with sponsors to tailor the best solution for each project and to deliver clinical trials to the highest quality. Reduce your R&D costs by outsourcing all your clinical research services under one provider.
George Clinical offers a full range of clinical trial implementation services across the Asia-Pacific region and the United States, with capabilities reaching into the EU and Latin America. Our expert teams work closely with sponsors to tailor the best solution for each project and to deliver clinical trials to the highest quality.
George Clinical is a leading contract research organization in Asia. We provide a comprehensive service to pharmaceutical, medical device and diagnostic customers, for registration trials and post-marketing trials. Our point of difference is the scientific leadership that we are able to access via our colleagues in The George Institute to ensure our clinical trial services achieve the highest scientific benchmarks.
Our highly experienced, locally-based teams allow us to deliver flexible and innovative solutions to accessing patient populations and ensure that our clinical trials make an impact for sponsors, clinicians and patients.
George Clinical has an exceptional track record in the efficient management and monitoring of clinical sites.
George Clinical provide expertise in:
George Clinical has taken a lead in the application of risk-based monitoring, the streamlined approach to monitoring clinical trials. The results generated by George Clinical are delivered in a timely fashion due to the central review of information. The multi-site comparisons and data centralization allows for the identification of outliers, the early identification of risks and the focused resolution of site issues. This methodology reflects George Clinical’s partner in research, The George Institute for Global Health’s experience in conducting pragmatic large scale projects with limited resources.
George Clinical are experts in:
Our Clinical Operations people are locally based in Australia, New Zealand, China, India, Korea, Hong Kong, Taiwan, Malaysia and the United States. In Europe, we have a growing team with locally-based people in the UK and Eastern Europe.
In some countries, such as Japan, we work with local partners to access suitably qualified and experienced Clinical Operations people who can join the George Clinical project teams.
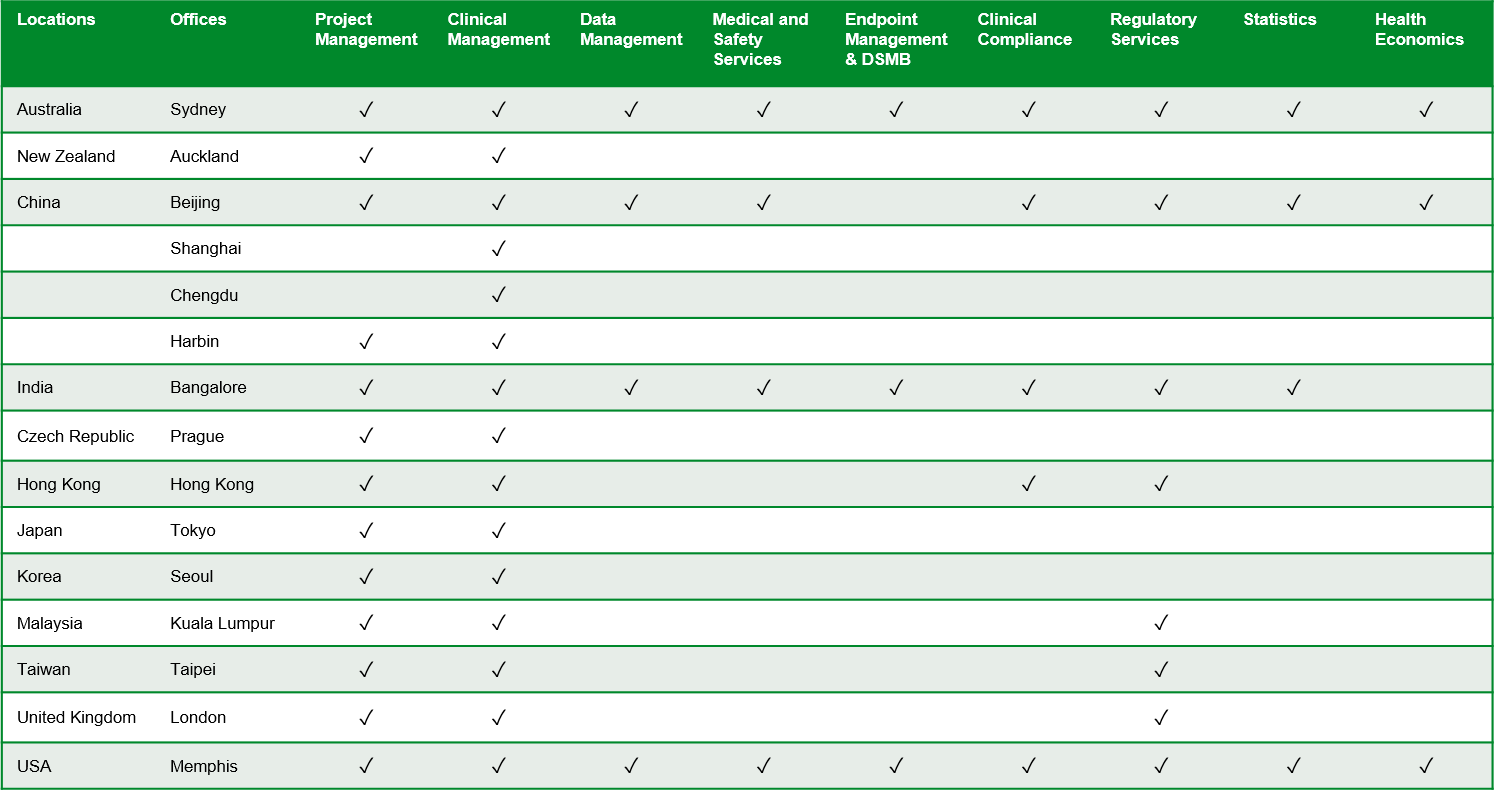
The table below depicts our service offerings by location.