The George Clinical statistics department operates globally to provide data and expertise that will enable faster, more informed decisions with reliable and flexible service. The statistics team works closely with the data management team to render an integrated and seamless data solution. Our services are flexible to cater to full-service studies or a strategic bespoke solution aligned to client organization needs.
The biostatistics team has extensive experience in the design and analysis of clinical trials in a wide range of therapeutic areas.
Solutions are tailored to customers’ requirements include Phase I – IV Clinical Trials, Late Phase / Real World studies, Regulatory Submissions, as well as complex surveys and longitudinal studies.
We support clinical trials and other research projects from conception to publication. All the analyses use up-to-date and validated software (e.g. SAS and Stata for statistical analyses, PASS for sample size calculations, and nQuery) and are performed to ICH GCP standards with emphasis on validation, traceability and reproducibility.
George Clinical’s statistics experts analyze and design clinical trials across a broad range of therapeutic areas, including, but not limited to: Oncology, Cardiology, Neurology, Respiratory, Nephrology, Endocrinology, Pain Control, Infectious Diseases, Critical Care, Substance Abuse and a diversity of Medical Devices.
To see the breadth of our networks in each therapeutic area, please click on the below links:
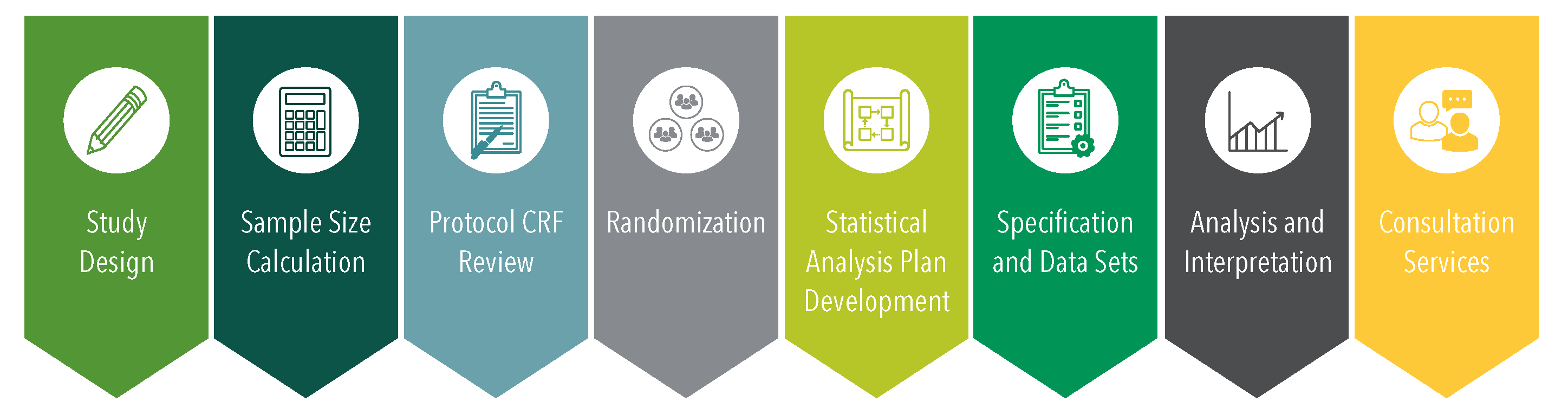
George Clinical biostatistics department’s key outputs include:
Stephen Jan is Head of the Health Economics and Process Evaluation Program at the George Institute for Global Health and Professor, Faculty of Medicine, UNSW Sydney.
He is an Honorary Professor at the University of Sydney, a Director of the Sax Institute and an Associate at both the Menzies Centre for Health Policy and the Poche Centre for Indigenous Health. He is a current NHMRC Principal Research Fellow and has previously held posts at the London School of Hygiene and Tropical Medicine and the Centre for Health Economics Research and Evaluation (CHERE) in Sydney. Stephen has over 20 years of experience in health economics, has published over 200 scientific articles and authored two textbooks in health economics.
He has worked closely with various governments of different levels, both in Australia (Commonwealth and State) and overseas, with international agencies such as the WHO and industry. His areas of expertise are economic evaluation, health financing, health sector priority setting, Indigenous and global health issues and the economics of chronic disease.
Laurent is a senior biostatistician with 20 years of experience in health research. He is Director of the Statistics Division at the George Institute for Global Health and Associate Professor, Faculty of Medicine, UNSW Sydney.
He is responsible for providing statistical services to the George Institute and its collaborators in Australia and globally. He holds a Master of Science in Statistics and Computer Science and a Master of Research in Public Health (Biostatistics). He is an accredited statistician by the Statistical Society of Australia (AStat).