

The dedicated project team
In testament to George Clinical’s operational experience and ability, we recently completed a Chinese registration trial for a mid-sized global pharmaceutical with excellent results against challenging timelines.
Key to the success of the study was identifying enhancements to the protocol to improve patient recruitment, which had suffered earlier setbacks. This finally led to impressive recruitment timelines where the George Clinical team achieved First Patient In within 3 months of contract signature, the first 50% of patients within 7 months, and the remaining 50% within 8 months. In total, the project team achieved the major milestone of Last Patient In 3 months ahead of schedule, despite initial challenges.
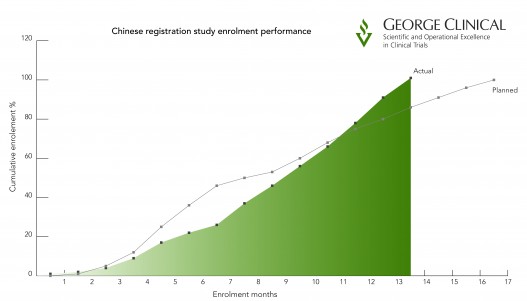
China registration study enrollment performance
Another key factor of success was close communication and collaboration with the sponsor to identify issues and resolutions early on the project. Recognizing that the project timelines were challenging, the Project Manager prepared efficient work plans for activities throughout the project. Excellent team work within the George Clinical project team, who had previously worked together on successfully delivering another global study in China, was also a driving force.
In recent years, George Clinical has seen significant growth, particularly in the Asia-Pacific region. With leadership and infrastructure invested in regional offices such as China, George Clinical is well positioned to provide tailored services for successful full service trials in China. Currently, George Clinical has over 40 personnel across 5 different functions, with offices in Beijing and Shanghai along with home based staff located in other key provinces.



