The Study
Although Obstructive sleep apnea (OSA) is a very common condition that is associated with an increased risk of cardiovascular disease (CVD), there is much uncertainty over the effectiveness of treatment with nasal continuous positive airway pressure (CPAP) for the prevention of CVD.
The SAVE trial was a multi-centre, open label, parallel, prospective, randomised, controlled trial of CPAP treatment plus standard care versus standard care alone, in 2717 high CVD risk subjects with moderate-severe OSA. The trial, funded by the National Health and Medical Research Council (NHMRC) of Australia, aimed to determine the effects of CPAP treatment over a two to seven year follow-up period on new cardiovascular events, including myocardial infarction, stroke and cardiovascular death.
The Save Trial China-Australia collaborative, multi-centre program of research was developed to establish the benefits of treatment of obstructive sleep apnea (OSA) on cardiovascular outcomes. There is currently relatively little community awareness of OSA in China compared to developed countries. Additionally access to treatment is severely restricted by cost – CPAP is currently beyond the means of nearly all sufferers of OSA. Thus the vast majority of cases in China currently go undetected and untreated.
Methods
SAVE was a multi-centre, open label, parallel, prospective, randomised, controlled trial of CPAP treatment plus standard care versus standard care alone, in high CVD risk subjects with moderate-severe OSA.
George Clinical’s China Component
George Clinical was engaged by the George Institute for Global Health to run the Project Management and
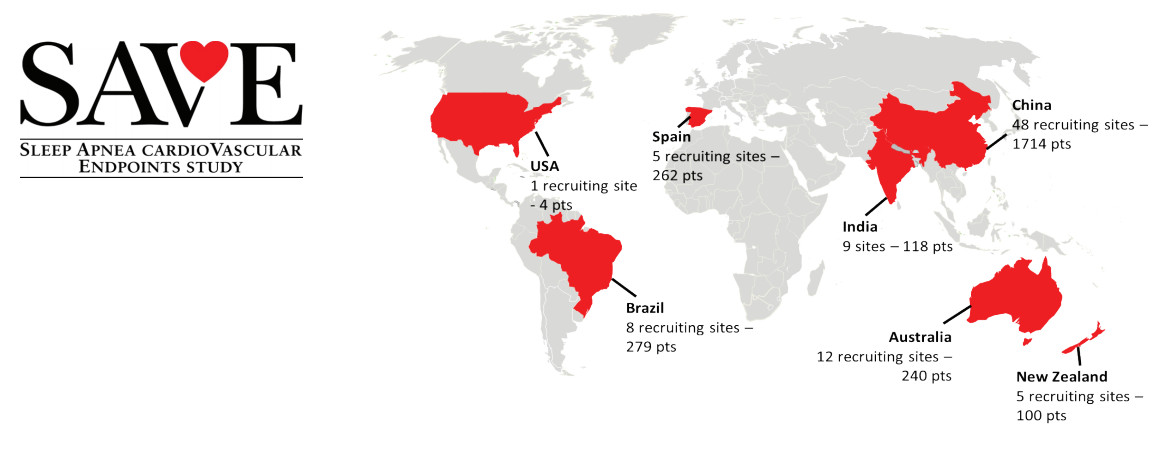
Site Monitoring operations for the China component of the SAVE Trial. George Clinical ran the trial over 48 sites, recruiting 1714 patients. George Clinical’s sites were the highest recruiting for the global study. Of the 2717 patients recruited, George Clinical’s China sites recruited 63% of total patients; Australia and New Zealand, the next best performing country, recruited just 12.5% of participants. 70% of George Clinical’s China sites were in the top ten recruiting sites for the entire study.
Results
In a large group of adults with both cardiovascular disease and moderate-to-severe obstructive sleep apnea, the use of CPAP therapy had no significant effect on the prevention of recurrent serious cardiovascular events, despite significantly reduced sleepiness and other symptoms of obstructive sleep apnea and improved quality-of-life measures.
The quality of the data and study design were sufficient for an article published in the New England Journal of Medicine (NEJM).
About George Clinical
George Clinical is a leading independent Asia-Pacific based clinical research organisation (CRO) with global capabilities, differentiated by scientific leadership, innovation and extensive investigator networks. With staff operating in 15 countires, George Clinical provides the full range of clinical trial services to biopharmaceutical, medical device and diagnostic customers, for all trial phases, registration and post-marketing trials. George Clinical combines scientific and clinical leadership with expert trial delivery capability to create a distinctive world-class service.



