Rare diseases affect 350 million globally, but with few available treatments, time is of the essence.
If you or a loved one suffered from a rare disease for which there is no treatment or cure, and there was a drug that had shown potential to give you some relief—some hope—you would want to have access to that drug as quickly as possible. That’s what was happening when the HIV/AIDS epidemic suddenly started taking lives at a rapid pace in the 1980s. AIDS activists demanded something—anything—to address the issue. They called on the FDA to offer greater flexibility in getting drugs approved, and it opened up new possibilities in the realm of drug development. The FDA allowed researchers to focus on surrogate endpoints that showed evidence of clinical benefits. AZT, approved in 1987, was the first drug approval based on a surrogate endpoint—and that ultimately led to the highly effective HAART drug that has allowed HIV/AIDS patients to live out their normal life spans.
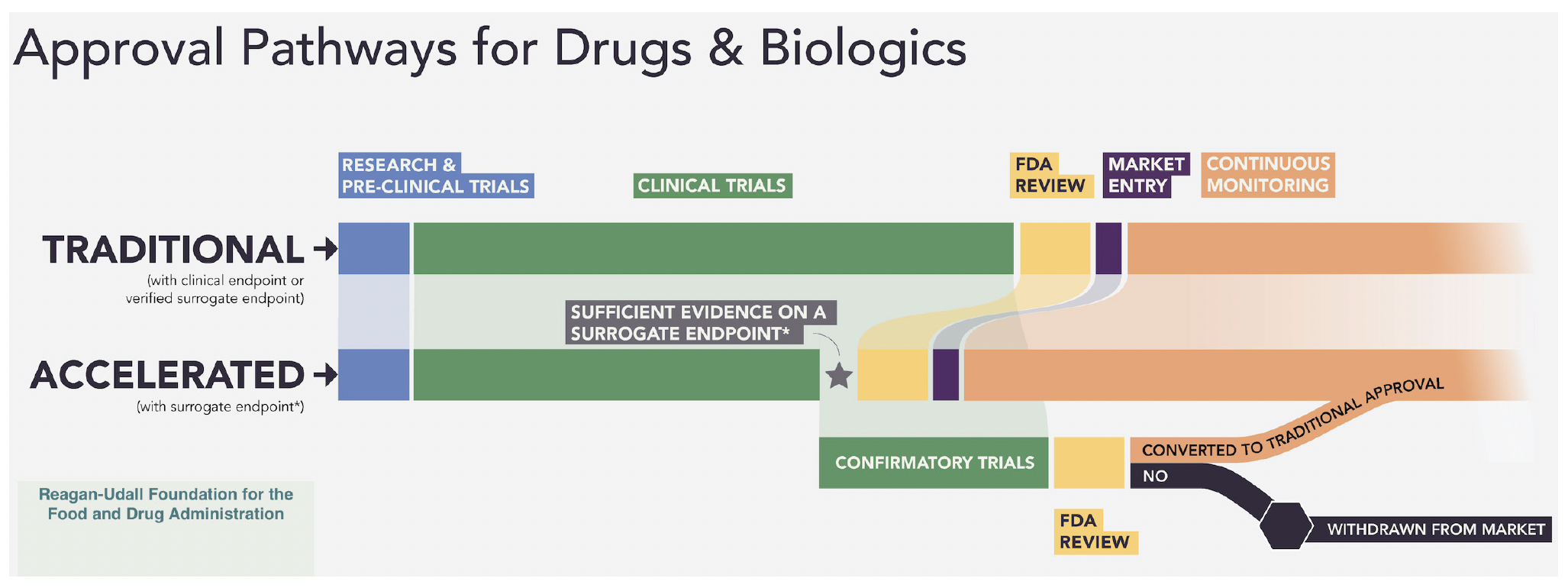
From that experience, the FDA created pathways for expediting drug development including the Accelerated Approval (AA) program initiated in 1992. AA provisions state that “the FDA may grant accelerated approval to a product for a serious or life-threatening disease or condition upon a determination that the product has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality (IMM) or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments.”
For drugs granted accelerated approval, postmarketing confirmatory trials are required to verify and describe the anticipated effect on IMM or other clinical benefit. As of December 31, 2023, 307 drugs have received accelerated approval, giving millions of patients reduction of disease symptoms, renewed hope and even extension of lifespan. As an example, for patients with non-small cell lung cancer (NSCLC) that had progressed or did not respond to prior therapy, two drugs that have received accelerated approval have resulted in earlier access for over 500,000 patients and nearly 200,000 additional years lived compared with expected survival had the drugs not been available.1
AA PROGRAM BENEFITS
Currently, greater than 80% of drugs in the AA program are for orphan indications for rare diseases—those that affect fewer than 200,000 people. In 1983, the Orphan Drug Act was passed, recognizing the fact that there were more than 20 million patients suffering from rare diseases that were being left out of scientific advancements in disease therapies. When patients came to testify in the congressional hearings—many of them children, Representative Henry A. Waxman, instrumental in passing this act stated, “It was as if someone had pulled back a curtain to reveal an entire segment of society that no one knew was there. Gathered together in a congressional hearing room before the national media were human beings with diseases so disabling or disfiguring that they never came out in public.”2
The passing of the Orphan Drug Act removed barriers for pharmaceutical companies and provided them financial incentives to research these diseases. There are >7,000 recognized rare diseases affecting nearly 350 million worldwide, and more than 90% do not have FDA-approved therapies. Nearly 80% of AA drugs are for rare cancers, which according to the International Agency for Research on Cancer (IARC), have fewer than six newly diagnosed cases per 100,000 people per year. Although individually these cancer types are rare, taken as a whole, they account for about 25–30% of all cancer diagnoses and 25% of cancer deaths.
The benefits of the AA program are undeniable. Where a traditional drug approval process can take as many as 15 years, drugs in the AA program, on the average, are available more than three years earlier. Earlier access to treatments for patients, smaller more efficient trials that take far less time to conduct, and a potential faster path to even better drugs make the AA program valuable.
WHAT ABOUT RISKS?
All aspects of new drug development come with risks, and the AA program is not an exception. Can patients be exposed to a drug that is ineffective or even unsafe? Do the smaller, shorter trials collect enough information on rare or delayed adverse effects? What if postmarketing confirmation is not done in a timely manner—or not at all? Will the high cost of these drugs mean that they get to only a few of the patients who need them? Will patients who are needed in future clinical trials be reluctant to join if they are already taking an accelerated approved drug? All of these risks have consequences. In some cases, the solution is determining risk vs. reward. In others, the risk can be mediated.
Of all of these issues, perhaps compliance with the postmarketing confirmation requirement has been the most controversial. Research has oft and clearly documented the slow and sometimes non-existent progress of these studies, with some AA drugs staying on the market for years without confirmation—all the while earning the drug company millions. One such drug was found to have been on the market for 25 years without completing its confirmatory studies while earning more than $200 million. In response to the issue, Congress has empowered the FDA to set more stringent requirements and timelines for these confirmation trials. There have been updates in 2023 and more reforms are being proposed.
Organizations such as the National Organization for Rare Disorders (NORD) who fully support the AA program are working with Congress and HHS to ensure that changes ultimately benefit the rare disease community—that’s 30 million Americans and 350 citizens worldwide who are waiting and hoping. And a recent analysis3 of the AA program determined that it is overall very successful, with half of all AA approvals converted to traditional approval in an average of little more than three years.
CONCLUSION
The AA program has proven to have tremendous benefits for people whose diseases are understudied and lack effective treatments. The drugs being given accelerated approval are held to the same gold standard as those in traditional approval and there is no question that these treatments are changing lives—not just for cancer patients, but also for kidney patients whose disease trajectory ultimately leads to dialysis or death. Before the FDA was prepared to consider urine protein-to-creatinine ratio (UPCR) as a surrogate endpoint, there were only one or two Phase II trials for IgA Nephropathy (IgAN) and no Phase III trials. Since UPCR was accepted there are now >150 Phase II and Phase III trials in progress and the first ever IgAN drugs have received accelerated approval status.
Tarpeyo (budesonide) was granted accelerated approval in December 2021 and followed with full approval in December 2023 based on clinical trial results for treatment of adults with primary IgAN. George Clinical is proud to have been a part of the PROTECT study that resulted in Filspari, the first non-immunosuppressive therapy for the reduction of proteinuria in IgAN, being granted accelerated approval in February 2023. The ongoing Phase III global, randomized, multicenter, double-blind, active-controlled clinical trial is designed to demonstrate whether Filspari slows kidney function decline. Topline results from the two-year confirmatory endpoints are expected in the fourth quarter of 2023 and are intended to support traditional approval of the drug.
These are proof that accelerated approval can function in the way it was intended, given the collaboration and partnership of sponsors, scientific leadership, trial managers and the FDA to ensure best practices. These approvals have built confidence in starting to look at other kidney diseases such as focal segmental glomerulosclerosis (FSGS) that could benefit from this approach.
As the science of drug development evolves, the AA program will need to make adjustments, and there are many invested experts willing to work closely with the FDA to ensure success. Industry needs to do their part by designing trials that account for the use of accelerated approval drugs and that can efficiently move into confirmatory studies. More education and stronger labeling is also needed for physicians and patients to explain what accelerated approval is and identify the drugs as such.
The goal is to keep drug development advancing, especially for rare diseases, so that lessons learned from early treatments generate even better solutions and more options for future patients. Accelerated approval is a critical cog in the drug development ecosystem to provide access to new, safe and effective drugs where there is currently no meaningful alternative. Patients who are suffering are waiting and hoping. And if you or a loved one is one of those patients, accelerated approval could literally change the trajectory of your life.
1 Partnership for Health Analytic Research, Issue Brief 7//26/22, Clinical Benefits of Accelerated Approval.
2 Institute for Clinical and Economic Review (ICER), The Next Generation of Rare Disease Drug Policy: Ensuring Both Innovation and Affordability, April 7, 2022.
3 Beakes-Read G, Neisser M, Frey P, Guarducci M. Analysis of FDA’s Accelerated Approval Program Performance December 1992–December 2021. Ther Innov Regul Sci. 2022 Sep;56(5):698-703. doi: 10.1007/s43441-022-00430-z. Epub 2022 Jul 28. PMID: 35900722; PMCID: PMC9332089.



